Researchers at the University of Illinois at Urbana-Champaign have developed a powerful new platform of polyureas that enable systems that are hydrolyzable, self-healing, and malleable, opening exciting applications in the packaging, coating, and adhesives industries. In this new class of polyureas, a bulky substituent is attached to one of the nitrogen atoms of the urea bond and destabilizes the typically stable linkage leading to dynamic dissociation. The polymers can be built from the vast library of isocyanate monomers already developed for the polyurea and polyurethane industries, yielding a wide range of materials with varying, engineered characteristics.
Hydrolyzable Polyureas (TF14085)
Hydrolyzable polymers are currently used in the agriculture and food industries as environmentally friendly plastics and packaging materials and in the biomedical field as drug delivery systems, surgical sutures, and scaffolds for tissue engineering. However, most hydrolyzable polymers, based on polyester materials or materials containing anhydride, acetal, ketal, or imine linkages, are synthesized via condensation or ring-opening polymerizations, often requiring high temperature conditions and catalysts and resulting in by-products. In contrast, because polyureas are made via a simple and clean chemistry without catalysts or by-products, hydrolyzable polyureas offer users the ability to tune polymer systems to specific applications without involving complex chemistries.
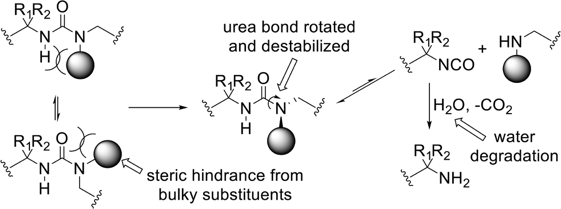
In this platform of polyureas, the destabilization introduced by the bulky substituent on the nitrogen of the urea bond leads to dynamic dissociation to the corresponding amines and isocyanates, with the isocyanates further undergoing irreversible hydrolysis in aqueous solution and complete degradation of the polyurea.
These degradable polymers are easily synthesized by reacting multifunctional bulky amines with isocyanates and exhibit tunable degradation kinetics.
Hydrolyzable Polyureas Bearing Hindered Urea Bonds
Hanze Ying and Jianjun Cheng
Journal of American Chemical Society 2014 136 (49), 16974-16977
Polyureas That Love to Break Down
Chemical and Engineering News, December 8, 2014, http://cen.acs.org/articles/92/i49/Polyureas-Love-Break-Down.html
Self-Healing Polyureas (TF12136)
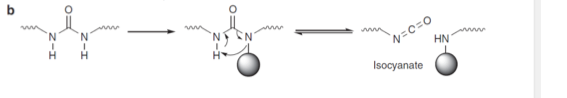
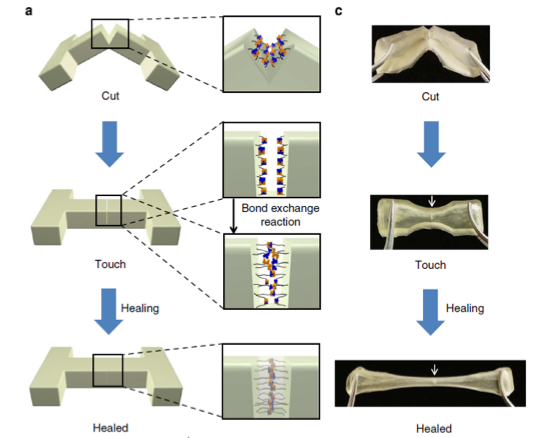
Incorporating the appropriate bulky substituents into polyureas enables polymers that can undergo autonomous, catalyst-free repair at room/low temperatures without the use of microcapsule healing agents, special precursors or customized laboratory/environmental conditions. Commercially available materials are used to produce self-healing polymers with capabilities to re-heal multiple times.
Here the bulky substituents are chosen so that (i) the dissociation and reverse (polymer-forming) reactions are rapid and (ii) the polymer-forming reaction is highly favored, thereby insuring optimum bulk mechanical properties of the polymer. Conventional polyureas and poly(urethane-urea)s can thus readily be made dynamic and self-healing while maintaining their stability by replacing regular amines with amines containing bulky substituents. By tuning the substituent, the dynamic properties of the polymer and its mechanical properties can be controlled.
Dynamic Urea Bond for the Design of Reversible and Self-Healing Polymers
Hanze Ying, Yanfeng Zhang, and Jianjun Cheng
Nature Communications 2014 5:3218 doi: 10.1038/ncomms4218
Also see.
Malleable Polyureas (TF15006 / 2015-152)
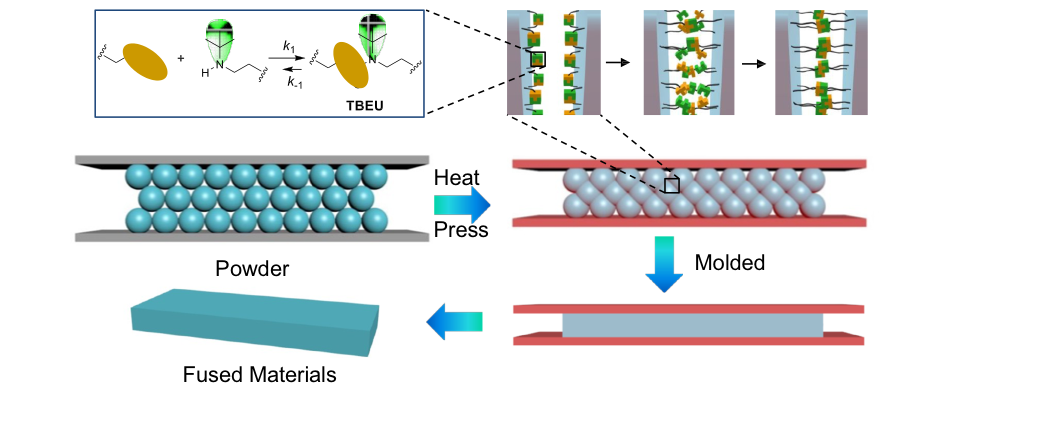
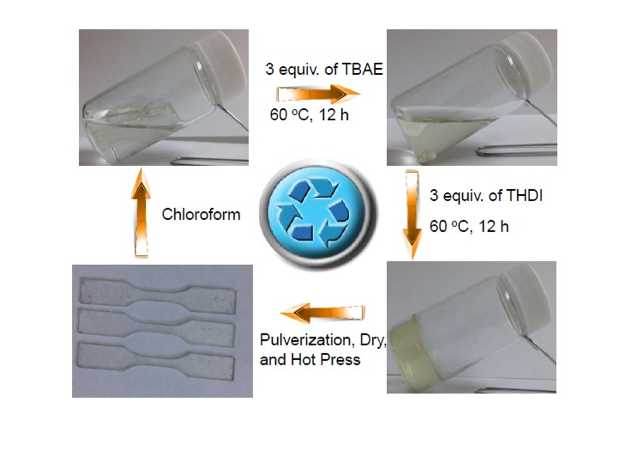
The polyurea systems can also be made malleable and recyclable by exploiting the reversible nature of the urea bond with the bulky subsituent. The cross-linked systems bearing the bulky substituents can be ground into powders and pressed/molded as shown below.
The polyurea systems can also be recycled as shown below.